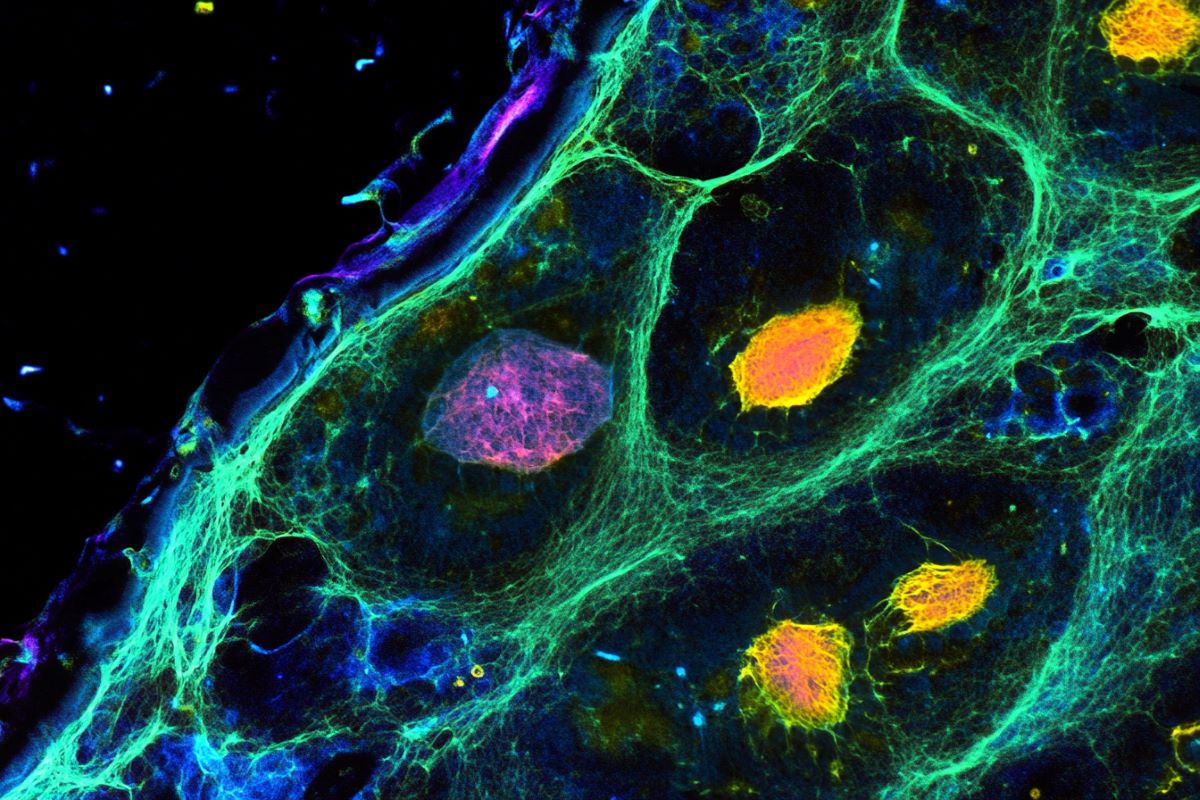
Summary: A study reveals how brain cell interactions influence aging, showing that rare cell types either accelerate or slow brain aging. Neural stem cells provide a rejuvenating effect on neighboring cells, while T cells drive aging through inflammation. Researchers used advanced AI tools and a spatial single-cell atlas to map cellular interactions across the lifespan

Navigating the Complex Regulatory Landscape for AI/ML-Enabled Medical Devices
The rapid advancement of Artificial Intelligence (AI) and Machine Learning (ML) is revolutionizing the healthcare industry. However, as these innovative devices emerge, understanding the complex regulatory landscape becomes crucial. This article delves into the key considerations for navigating FDA regulations for AI/ML-enabled medical devices.
Understanding AI/ML in Healthcare
AI and ML are being used to derive new insights from vast amounts of healthcare data, leading to significant advancements in various areas:
- Predictive Analytics: AI algorithms can analyze patient data to predict disease risk, optimize treatment plans, and improve patient outcomes.
- Image Analysis: AI-powered image analysis tools can aid in the early detection and diagnosis of diseases by analyzing medical images like X-rays, MRIs, and CT scans.
- Drug Discovery: AI can accelerate drug discovery by identifying potential drug targets and optimizing clinical trial design.
- Personalized Medicine: AI can analyze patient-specific data to tailor treatment plans and improve therapeutic outcomes.
FDA Regulation of AI/ML-Enabled Medical Devices
The FDA has been actively working to adapt its regulatory framework to accommodate the rapid evolution of AI/ML-enabled medical devices. Key considerations include:
- Premarket Review: Devices incorporating AI/ML may require premarket review through 510(k), De Novo, or Premarket Approval (PMA) pathways, depending on their risk classification and intended use.
- Software as a Medical Device (SaMD): FDA has specific regulations for SaMD, including criteria for determining whether software is subject to device regulations.
- Real-World Evidence (RWE): FDA may require RWE to assess the long-term safety and effectiveness of AI/ML-enabled devices.
- Algorithmic Bias and Fairness: Addressing bias in AI algorithms is essential to ensure equitable and effective healthcare.
- Transparency and Accountability: Manufacturers should be transparent about the development, validation, and performance of AI/ML algorithms.
Key Challenges and Opportunities
While AI/ML offer significant potential benefits, there are challenges to overcome:
- Regulatory Uncertainty: The evolving regulatory landscape can create uncertainty for manufacturers.
- Data Quality and Privacy: Ensuring data quality and protecting patient privacy are critical considerations.
- Algorithm Validation and Testing: Rigorous validation and testing are necessary to ensure the safety and effectiveness of AI/ML-based devices.
- Ethical Considerations: Addressing ethical implications, such as algorithmic bias and transparency, is essential.
As Nate Downing, Managing Attorney at Gardner Law, emphasizes, “The intersection of AI/ML and healthcare presents both immense opportunities and significant regulatory challenges. Navigating this complex landscape requires a deep understanding of FDA regulations, a commitment to ethical development, and a focus on patient safety and efficacy.”
By proactively addressing these challenges and working closely with regulatory authorities, manufacturers can successfully navigate the regulatory landscape and bring innovative AI/ML-enabled medical devices to market.