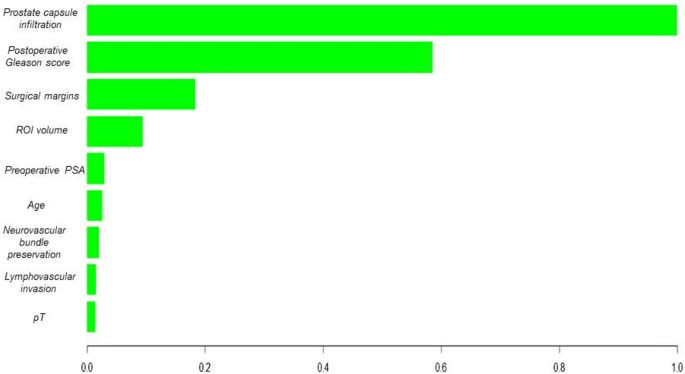
Introduction Prostate cancer (PCa) is a major public health issue in developed countries1. Radical prostatectomy (RP) is associated with a lower incidence of disease progression than active monitoring2,3. However, one-third of patients experience biochemical recurrence (BCR) after the procedure4,5. Previous studies have shown that the survival rate of patients with BCR is 20% lower after

European Commission issues CE mark for Zeiss CIRRUS PathFinder AI tool
The clinical support system integrates artificial intelligence into OCT interpretation workflow

The all-in-one, AI-enhanced tool eliminates need for data exports or third-party platforms, according to Zeiss Medical Technology. Image credit: © olrat – stock.adobe.com

In a press release, Zeiss Medical Technology (Carl Zeiss Meditec AG) announced the European Commission has issued a CE mark for its CIRRUS PathFinder clinical support too. The CIRRUS PathFinder uses artificial intelligence (AI) technology to support imaging and the clinical workflow. According to the press release from the company, a proprietary deep learning algorithm accelerates optical coherence tomography (OCT) interpretation.1 Along with automatically identifying anomalous or abnormal OCT-B scans, a licensed element of the new CIRRUS software release, the platform also provides AI-enhanced OCT angiography (OCTA) image quality and multilayer segmentation.
Zeiss Medical Technology described1 the clinical applications of the Cirrus PathFinder platform, which “delivers high-speed image capture with HD imaging detail, a wide field of view, and AI decision support.” The all-in-one nature of the clinical support tool eliminates data exports or analysis with third-party platforms. The AI tool flags areas of concern during image acquisition, highlighting areas which may require more detailed follow-up imaging. Additionally, the software uses AI to enhance visualisation of the vascular structure. Enhanced layer segmentation and B-scan averaging of OCTA structural scans also provide more visibility into the structure of the eye.
Euan S. Thomson, PhD, head of the digital business unit for Zeiss Medical Technology, commented1 on the approval. “ZEISS is harnessing the power of data and artificial intelligence to deliver integrated digital solutions that are paving the way for the next era in ophthalmic care,” he said. “Our strategic focus on digitally connected workflows has placed us at the forefront for enabling ophthalmologists with powerful AI-driven capabilities, like those that have been fully integrated into Zeiss CIRRUS PathFinder.”
Magnus Reibenspiess, head of the ophthalmology strategic business unit at Zeiss Medical Technology, also provided comment.1 “The AI decision support capabilities of Zeiss CIRRUS PathFinder help enable faster, more informed and actionable diagnostics for a better clinical and patient experience,” he said.
The approval reflects an increased emphasis on AI-supported workflows and integrations at Zeiss Medical Technology. In May, the company introduced its new Zeiss Research Data Platform (ZEISS RDP), a cloud-based, AI-driven solution.2 Additionally, the Visumax 800 femtosecond laser with SMILE pro software received NMPA approval in China at the beginning of 2025.3 The surgical suite includes workflow enhancements such as VISULYZE nomograms for securely collecting and analysing patient data.
References
-
ZEISS announces CE mark for CIRRUS PathFinder AI tool with automated OCT assessment. Press release. Carl Zeiss Meditec AG. Published August 21, 2025. Accessed August 21, 2025.
-
Harp MD. ZEISS debuts Research Data Platform, highlights Boehringer Ingelheim partnership. Ophthalmology Times Europe. Published May 2, 2025. Accessed August 21, 2025. https://europe.ophthalmologytimes.com/view/zeiss-debuts-research-data-platform-highlights-boehringer-ingelheim-partnership
-
Hayes H. Zeiss Visumax 800 with SMILE pro software receives NMPA approval in China. Ophthalmology Times Europe. Published February 26, 2025. Accessed August 21, 2025. https://europe.ophthalmologytimes.com/view/zeiss-visumax-800-with-smile-pro-software-receives-nmpa-approval-in-china